On 13 February 2020 the FSA set a new deadline of 31 March 2021 for all CBD companies to have scientific dossiers for each of their products validated for Novel Foods authorisation. After this date, only CBD products with a validated Novel Foods dossier will be allowed to be sold online and in shops in the UK.
The purpose of this timeline is to highlight the importance that CBD companies must start this process now in order to avoid disruption in their distribution channels when these regulations come into force.
Novel Foods CBD dossier overview
To build a credible Novel Foods dossier a CBD company must collate data to demonstrate to EFSA (upto 31 December 2020), and the FSA, that their products are safe for human consumption. Once EFSA is satisfied that enough information is provided they will be able to validate the dossier. Once validated, the dossier will then go through the authorisation process. To remain on the shelves in the UK on 1 April 2021 a product needs to be validated.
Each CBD product dossier is made up of information relating to the product itself, for example the production process or compositional data. It will also include data that needs to be generated. Of these two types of data, it is the generated data that companies need to be conscious of now.
Submission deadlines
Once a dossier is submitted to EFSA and FSA it will take 2-3 months to be validated, therefore companies should submit dossiers to EFSA (with FSA in copy) by October 2020 or by December 2020 at the very latest to be validated by 31 March 2021.
After 1 January 2021, Novel Foods and Brexit
According to FSA “If your product or process has been authorised by the European Commission and the necessary legislation is in place and applies by the end of the transition period, that authorisation will remain valid in the UK.”
Where to submit a Novel Foods dossier
Up to 31 December 2020
For authorisation in the UK and EU – submit to FSA and EFSA
After 1 January 2021
For authorisation in the UK – submit to FSA
For authorisation in the EU – submit to EFSA
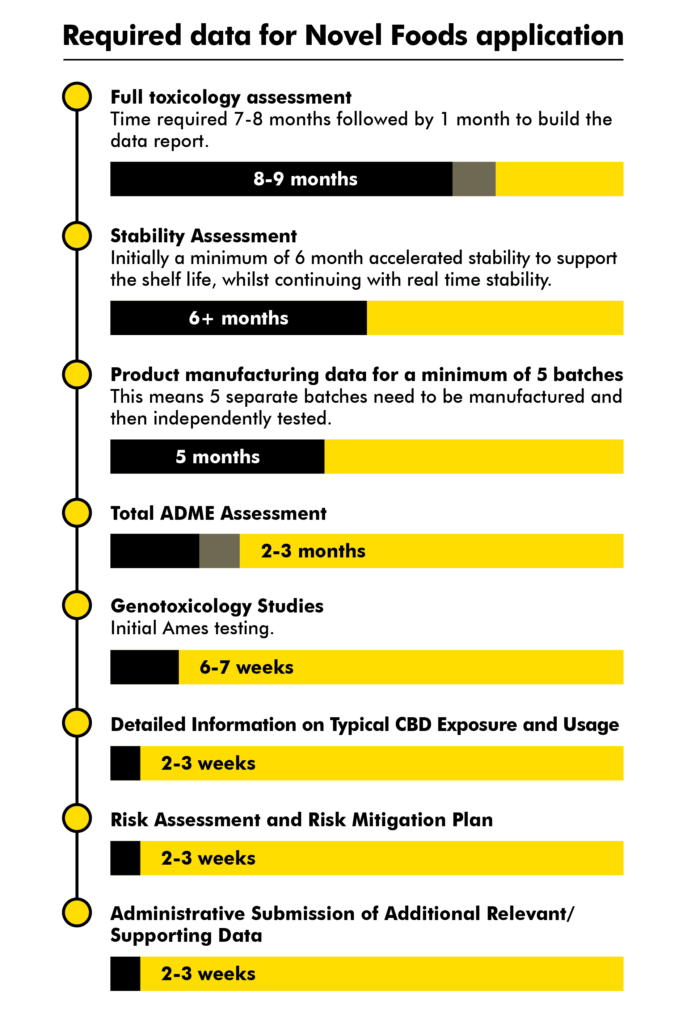
Timeline of required data for Novel Foods application
Here is the data that will need to be included in the dossier followed by an estimate of time to produce this data. These can be gathered in parallel.
Date to start full Novel Foods CBD dossier application process
Given the soft deadline of October 2020 to submit a full CBD dossier for each product (to allow EFSA the time to validate a CBD dossier before the UK leaves EU and in time for FSA’s 31 March 2021 hard deadline) a company should have started the Toxicology Assessment (total 9 months) by 31 January 2020.
A company that has not started this process may be concerned that this date is in the past. However, it is possible that they could use the data from their CBD supplier, provided the supplier intends to submit a Novel Foods application. To find out if this is the case, The ACI have compiled 5 questions for a CBD company to ask their supplier.
5 questions for a CBD company to ask their supplier
- Is your CBD supplier submitting a Novel Foods application for CBD?
- What is the estimated time scale for their submission?
- Are they covering your manufacturing details for the formulation of your finished product in their Novel Foods application?
- In the case of IP issues, you should request the generic finish formulation procedure. For example, safety, stability and recommended dose status. This is to risk mitigate your seed to shelf methodology.
- Establish your CBD manufacturer’s/supplier’s accreditation status (ISO or cGMP, GACP, HACCP)
Once it is established that the CBD raw material supplier is submitting a Novel Foods application this makes the application process simpler for brands and manufacturers, as they are now only responsible for risk assessment (2-3 weeks) and stability assessment (6+ months) of their finished product.
The ACI will guide our members through this process and help them ensure that they submit credible Novel Foods CBD dossiers.
Contact us for membership information.
Useful links
- FSA CBD deadline announcement https://www.food.gov.uk/news-alerts/news/food-standards-agency-sets-deadline-for-the-cbd-industry-and-provides-safety-advice-to-consumers
- FSA CBD guidance https://www.food.gov.uk/business-guidance/cannabidiol-cbd
- FSA Novel Foods Guidance https://www.food.gov.uk/business-guidance/novel-foods
- Submitting a regulated product authorisation application from 1 January 2021 https://www.food.gov.uk/business-guidance/submitting-a-regulated-product-authorisation-application-from-1-january-2021
- EFSA Novel Foods applications https://ec.europa.eu/food/safety/novel_food/authorisations_en

